New Nickel Catalyst Converts Carbon Dioxide into Sustainable Hydrocarbon Fuels
A research team led by Associate Professor Boon Siang Yeo has developed a nickel-based catalyst that efficiently converts carbon dioxide into valuable liquid hydrocarbons, essential components of fuels like gasoline and jet fuel. By fine-tuning the catalytic process, the team achieved unprecedented control over the production of branched hydrocarbons, which burn more efficiently and offer higher performance. This breakthrough opens new pathways for sustainable fuel production.
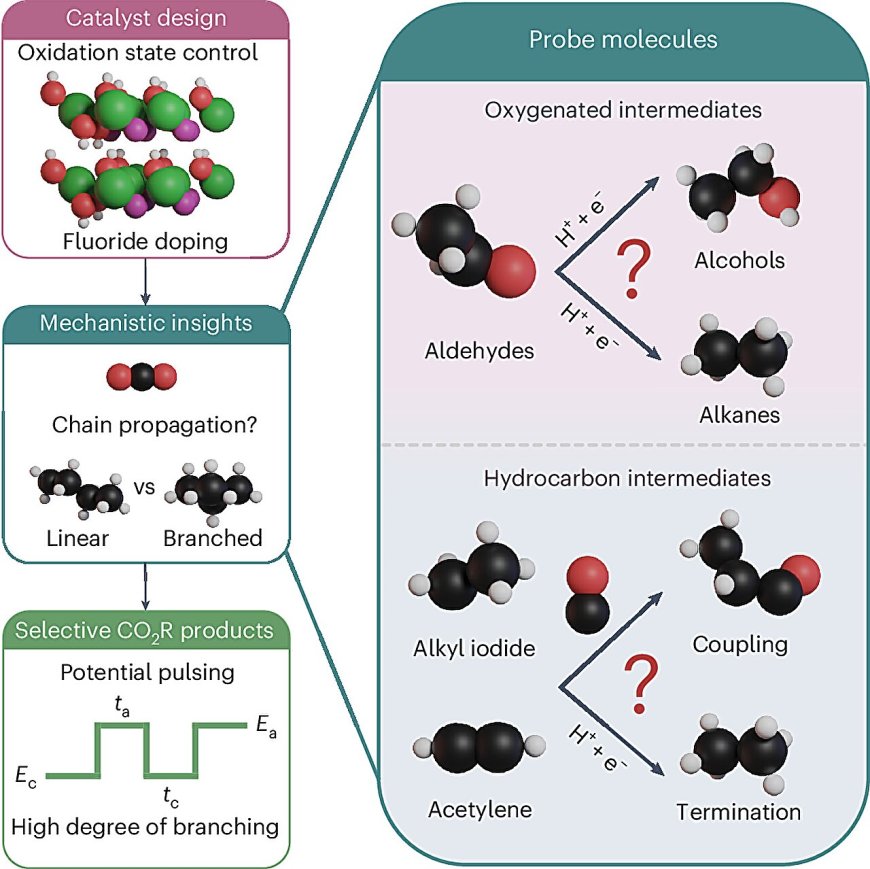
A research team led by Associate Professor Boon Siang Yeo from the Department of Chemistry at National University of Singapore (NUS) has developed a new way to convert carbon dioxide into valuable liquid hydrocarbons, key components of fuels like gasoline and jet fuel. The study, published in Nature Catalysis, involved collaboration with experts in computational simulation and catalytic fuel synthesis.
Scientists have traditionally used copper as a catalyst to convert carbon dioxide into simpler products. However, copper has limitations in producing longer, branched hydrocarbon chains essential for high-quality fuels. The team explored using a nickel-based material and innovative strategies to fine-tune the catalytic process, achieving unprecedented control over the types of hydrocarbons produced.
By introducing fluoride ions into the nickel structure and applying pulsed potential electrolysis, the team significantly increased the production of branched hydrocarbons. This advancement is crucial for creating more efficient and high-performance fuels suitable for vehicles and aircraft.
The study's findings offer new insights into the behavior of nickel and copper catalysts at the molecular level, highlighting the advantages of nickel-based catalysts in promoting the formation of longer hydrocarbon chains. The research paves the way for the development of sustainable aviation fuels and chemical precursors, supporting the global transition to cleaner technologies.
According to the source: Phys.org.
What's Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0



















































































































































































